ผู้ใช้:M sky/เอนไซม์
เอนไซม์ (อังกฤษ: Enzyme) คือโมเลกุลชีวภาพที่ช่วยเร่งปฏิกิริยาเคมีในสิ่งมีชีวิต[1][2] โดยทั่วไปเอนไซม์เป็นสารประเภทโปรตีน แต่เอนไซม์ก็ยังมีส่วนประกอบของโมเลกุลอาร์เอ็นเอ (RNA) ซึ่งมีคุณสมบัติในการเร่งอัตราปฏิกิรยาเคมีชีวภาพด้วย ในเวลาต่อมาโมเลกุลอาร์เอ็นเอเหล่านี้เป็นที่รู้จักกันในชื่อไรโบไซม์ (ribozymes)[3] ในปฏิกิริยาชีวภาพที่มีเอนไซม์ร่วมด้วยนั้น โมเลกุลของสารตั้งต้น (Substrate) จะถูกเอนไซม์เปลี่ยนสภาพเป็นโมเลกุลที่เปลี่ยนไป เรียกว่า ผลิตภัณฑ์ (Products) กระบวนการส่วนใหญ่ในเซลล์ของสิ่งมีชีวิตต้องการเอนไซม์เพื่อให้อัตราของปฏิกิริยาเกิดขึ้นอย่างเหมาะสม เนื่องด้วยคุณสมบัติของเอนไซม์ที่มีความจำเพาะ (selective) กับสารตั้งต้นและมีคุณสมบัติเร่งปฏิกิริยาของสิ่งมีชีวิต เอนไซม์เป็นสารที่สังเคราะห์ได้จากเซลล์และผ่านกระบวนการเมตาบอลิซึมในเซลล์
เอนไซม์มีคุณสมับติเหมือนตัวเร่งปฏิกิรยาเคมีทั่วไป เอนไซม์ทำงานด้วยลดพลังงานกระตุ้น (activation energy) (Ea or ΔG‡) ในปฏิกิริยาเคมี ดังนั้นจึงสามารถเพิ่มอัตราการเกิดปฏิกิริยาเคมีได้อย่างมหาศาล เอนไซม์ส่วนมากสามารถเร่งปฏิกิริยาได้เร็วขึ้นประมาณ 1 ล้านเท่าเมื่อเปรียบเทียบกับปฏิกิริยาที่ไม่มีตัวเร่งปฏิกิริยา และเอนไซม์ก็ยังมีคุณสมบัติเหมือนตัวเร่งทั่วไปอีก กล่าวคือ เอนไซม์จะไม่สุญสลายไปกับปฏิกิริยาหรือเปลี่ยนรูปเป็นผลิตภัณฑ์ใหม่ อย่างไรก็ตาม เอนไซม์มีคุณสมับติที่ต่างจากตัวเร่งปฏิกิริยาทั่วไปคือ เอนไซม์มีความจำเพาะกับสารตั้งต้น ปัจจุบันนักวิทยาศาสตร์ค้นพบปฏิกิริยาที่มีเอนไซม์เป็นตัวเร่งในปฏิกิรยาชีวภาพกว่า 4,000 ปฏิกิริยา[4] ในส่วนของโมเลกุลอาร์เอ็นเอซึ่งเรียกว่าไรโบไซม์ มีส่วนร่วมกับการเร่งปฏิกิริยาในไรโบโซมเป็นสำคัญ [5][6] การสังเคราะห์โมเลกุลหรือเอนไซม์สังเคราะห์ (artificial enzyme) ได้ผลลัพธ์เอนไซม์คล้ายตัวเร่งปฏิกิริยาทั่วไป[7]
กิจกรรมของเอนไซม์สามารถหยุดยั้งได้ด้วยตัวยับยั้งเอนไซม์ (enzyme inhibitor) ซึ่งเป็นโมเลกุลที่ลดกิจกรรมของเอนไซม์ ตัวเร่งเอนไซม์คือโมเลกุลที่ช่วงส่งเสริมกิจกรรมของเอนไซม์ ยาและยาพิษส่วนมากเป็นตัวยับยั้งเอนไซม์ นอกจากนี้ยังมีปัจจัยอื่นที่เกี่ยวข้องกับการทำงานของเอนไซม์ ได้แก่ อุณหภูมิ ความเป็นกรด-เบส และความเข้มข้นของสารตั้งต้น เอนไซม์บางชนิดถูกนำมาใช้ในทางการค้า อาทิ การสังเคราะห์ยาปฏิชีวนะ นอกจากนี้ ผลิตภัณฑ์สำหรับบ้านยังได้นำเอนไซม์มาใช้เป็นส่วนประกอบในการเร่งปฏิกิริยาชีวเคมี เช่น การใช้เอนไซม์ในผงซักฟอกเพื่อทำความสะอาดคราบโปรตีนหรือไขมันในเสื้อผ้า เอนไซม์ในเครื่องเพิ่มความอ่อนนุ่มให้กับเนื้อวัว ซึ่งทำลายพันธะของโปรตีนช่วยให้เคี้ยวเนื้อได้ง่ายขึ้น เป็นต้น
นิรุกติศาสตร์และประวัติ[แก้]

ในช่วงระหว่างคริสต์ศศวรรษที่ 1700 และ 1800 มีการค้นพบการย่อยเนื้อ โดยสารคัดหลั่งจากกระเพาะ[8] และการเปลี่ยนแป้งเป็นน้ำตาลโดยสารสกัดจากพืชและน้ำลาย อย่างไรก็ตามกระบวนการที่ทำให้เกิดผลดังกล่าวไม่สามารถอธิบายได้[9]
ในคริสต์ศตวรรษที่ 19 ระหว่างการศึกษาการใช้ยีสต์ (yeast) หมักน้ำตาลเป็นแอลกอฮอล์ หลุยส์ ปาสเตอร์สรุปกระบวนการหมักว่าถูกเร่งปฏิกิริยาโดยเชื้อหมัก (ferments) ในยีสต์และคิดว่าสิ่งเหล่านี้เกิดขึ้นในสิ่งมีชีวิตเท่านั้น เขาบันทึกว่า "กระบวนการหมักแอลกอฮอล์เป็นกระบวนการที่มีความสัมพันธ์กับสิ่งมีชีวิตและการจัดองค์ประกอบในเซลล์ของยีสต์ โดยไม่พบในเซลล์ที่เน่าเปื่อยหรือตายแล้ว"[10]
ในปี ค.ศ. 1878 Wilhelm Kühne (1837 - 1900) นักสรีรวิทยาชาวเยอรมัน เป็นคนแรกที่ได้ใช้คำว่า "เอนไซม์" ซึ่งเป็นคำมาจากภาษากรีก (กรีก: ενζυμον) แปลว่า, "ในการหมัก" เพื่ออธิบายกระบวนการที่เกิดขึ้น คำว่า "เอนไซม์" ได้นำมาใช้ภายหลังเพื่อกล่าวถึงสารที่ไม่มีชีวิต อาทิ เปปซิน และคำว่า เชื้อหมัก (อังกฤษ: ferment) ได้นำมาใช้เพื่อกล่าวถึงกิจกรรมทางเคมีที่ผลิตโดยสิ่งมีชีวิต
ในปี ค.ศ. 1897 อี บุชเนอร์ (เยอรมัน: Eduard Buchner) เริ่มต้นการศึกษาความสามารถของสารหลั่งจากยีสต์ซึ่งมีจำกัดในเซลล์ของยีสต์ที่มีชีวิตเพื่อหมักน้ำตาล กระบวนการทดลองดังกล่าว ทำการทดลอง ณ มหาวิทยาลัยแห่งเบอร์ลิน เขาพบว่าน้ำตาลสามารถหมักต่อไปได้โดยปราศจากเซลล์ของยีสต์ที่มีชีวิต[11] เขาตั้งชื่อเอนไซม์ที่สกัดออกมาจากกระบวนการหมักนำ้ตาลซูโครสว่า "ไซม์เมส" (zymase)[12] ในปี ค.ศ. 1907 เขาได้รับรางวัลโนเบลสาขาเคมีสำหรับงานวิจัยทางชีวเคมีและค้นพบกระบวนการหมักที่ปราศจากสิ่งมีชีวิต ต่อมาได้มีการยึดถือแบบงานของบุชเนอร์เป็นต้นแบบ อาทิ การตั้งชื่อเอนไซม์ตามสิ่งที่เอนไซม์เข้าเร่งปฏิกิริยา โดยทั่วไปจะตั้งชื่อเอนไซม์ตามสารตั้งต้นตามด้วยคำว่า -เอส (-ase) เช่นเอนไซม์แล็กเตสเป็นเอนไซม์ที่ทำปฏิกิริยากับน้ำตาลแล็กโตส หรือชนิดของปฏิกิริยา เช่น ดีเอ็นเอโพลีเมอเรสทำให้เกิดดีเอ็นเอพอลิเมอร์
ดังที่กล่าวไปแล้วว่า เอนไซม์สามารถทำงานได้ภายนอกเซลล์ ขั้นตอนต่อไปคือการศึกษาธรรมชาติของสารชีวเคมีดังกล่าว นักวิทยาศาสตร์และนักวิจัยหลายคนได้บันทึกไว้ว่ากิจกรรมของเอนไซม์เกี่ยวข้องกับโปรตีน แต่นักวิทยาศาสตร์บางคน อาทิ Richard Willstätter นักวิทยาศาสตร์ผู้ได้รับรางวัลโนเบลโต้แย้งว่าโปรตีนเป็นเพียงตัวพาสำหรับเอนไซม์ที่แท้จริงและโปรตีน per se ไม่มีความสามารถในฐานะเป็นตัวเร่งปฏิกิริยา อย่างไรก็ตาม ในปี ค.ศ. 1926 James B. Sumner ได้แสดงให้เป็นว่าเอนไซมืยูรีเอสเป็นโปรตีนบริสุทธิ์และสามารถตกผลึกได้ ซัมเนอร์ทดลองกระบวนการโดยใช้เอนไซม์เป็นตัวเร่งในปี ค.ศ. 1937 บทสรุปคือโปรตีนบริสุทธิ์สามารถเป็นเอนไซม์ได้ ซึ่งพิสูจน์โดย John Howard Northrop และ Wendell Meredith Stanley นักวิจัยเรื่องเอนไซม์เปปซินในระบบย่อยอาหารในปี ค.ศ. 1930 รวมทั้งทริปซิน (trypsin) และไคโมทริปซิน (chymotrypsin) นักวิทยาศาสตร์ทั้งสามได้รับรางวัลโนเบลสาขาเคมีในปี ค.ศ. 1946[13]
การค้นพบการตกผลึกของเอนไซม์ทำให้สามารถค้นพบโครงสร้างของเอนไซม์ได้ในเวลาต่อมาโดยผ่ายกระบวนการตรวจโครงสร้างผลึกด้วยรังสีเอ็กซ์ (x-ray crystallography) กระบวนการนี้ได้ทำการทดลองกับไลโซโซมเป็นครั้งแรก ซึ่งเป็นเอนไซม์ที่พบได้ในน้ำตา น้ำลายและไข่ขาวซึ่งทำลายชั้นหุ้มแบคทีเรียบางชนิด โครงสร้างของเอนไซม์ดังกล่าวค้นพบโดยคณะทำงานของ David Chilton Phillips และตีพิมพ์ผลงานของเขาในปี ค.ศ. 1965[14] การค้นพบโครงสร้างของไลโซโซมทำให้เป็นจุดเริ่มต้นการศึกษาชีวโครงสร้าง และทำให้เข้าใจกระบวนการทำงานของเอนไซม์ในระดับอะตอมในเวลาต่อมา
โครงสร้างและกลไก[แก้]

โครงสร้างเอนไซม์โดยทั่วไปเป็นโปรตีนทรงกลม ประกอบด้วยอะมิโนตั้งแต่ 62 หน่วยขึ้นไปซึ่งพบได้ในโมโนเมอร์ของ 4-Oxalocrotonate tautomerase[15] จนถึงอะมิโน 2,500 หน่วยซึ่งพบในเอนไซม์สลายกรดไขมัน[16] A small number of RNA-based biological catalysts exist, with the most common being the ribosome; these are referred to as either RNA-enzymes or ribozymes. The activities of enzymes are determined by their three-dimensional structure.[17] However, although structure does determine function, predicting a novel enzyme's activity just from its structure is a very difficult problem that has not yet been solved.[18]
Most enzymes are much larger than the substrates they act on, and only a small portion of the enzyme (around 3–4 amino acids) is directly involved in catalysis.[19] The region that contains these catalytic residues, binds the substrate, and then carries out the reaction is known as the active site. Enzymes can also contain sites that bind cofactors, which are needed for catalysis. Some enzymes also have binding sites for small molecules, which are often direct or indirect products or substrates of the reaction catalyzed. This binding can serve to increase or decrease the enzyme's activity, providing a means for feedback regulation.
Like all proteins, enzymes are made as long, linear chains of amino acids that fold to produce a three-dimensional product. Each unique amino acid sequence produces a specific structure, which has unique properties. Individual protein chains may sometimes group together to form a protein complex. Most enzymes can be denatured—that is, unfolded and inactivated—by heating or chemical denaturants, which disrupt the three-dimensional structure of the protein. Depending on the enzyme, denaturation may be reversible or irreversible.
ความจำเพาะ[แก้]
เอนไซม์มีความจำเพาะต่อปฏิกิริยาที่เข้าไปเร่งและสารตั้งต้นที่เกี่ยวข้องกับปฏิกิริยา นอกจากนี้ยังมีปัจจัยอื่นๆอาทิ รูปร่างและประจุ คุณสมบัติที่เข้ากับน้ำได้ดี (hydrophilic) และคุณสมบัติที่ไม่เข้ากับน้ำ (hydrophobic) ที่เพิ่มคุณสมับติความจำเพาะของเอนไซม์และสารตั้งต้นที่เอนไซม์ทำปฏิกิริยา ซึ่งสามารถพบได้ในการศึกษาระดับความจำเพาะ 3 มิติ (stereospecificity]), บริเวณจำเพาะ (regioselectivity) และ เคมีจำเพาะ (chemoselectivity)[20]
Some of the enzymes showing the highest specificity and accuracy are involved in the copying and expression of the genome. These enzymes have "proof-reading" mechanisms. Here, an enzyme such as DNA polymerase catalyzes a reaction in a first step and then checks that the product is correct in a second step.[21] This two-step process results in average error rates of less than 1 error in 100 million reactions in high-fidelity mammalian polymerases.[22] Similar proofreading mechanisms are also found in RNA polymerase,[23] aminoacyl tRNA synthetases[24] and ribosomes.[25]
Some enzymes that produce secondary metabolites are described as promiscuous, as they can act on a relatively broad range of different substrates. It has been suggested that this broad substrate specificity is important for the evolution of new biosynthetic pathways.[26]
แบบจำลองลูกกุญแจและแม่กุญแจ[แก้]
เนื่องจากคุณสมบัติความจำเพาะของเอนไซม์ อีมิล ฟิชเชอร์ (Emil Fischer) ได้เสนอแบบจำลองลูกกุญแจแลพแม่กุญแจ (lock and key model) ขึ้นในปี ค.ศ. 1894 โดยอธิบายว่าเนื่องมาจากเอนไซม์และสารตั้งต้นมีรูปร่างของโมเลกุลที่สามารถเข้ากันและกันได้อย่างพอดี[27] เสมือนกับเอนไซม์เป็นลูกกุญแจและสารตั้งต้นเป็นแม่กุญแจที่สามารถเข้ากันได้อย่างพอดี อย่างไรก็ตาม แม้ว่าแบบจำลองนี้สามารถอธิบายคุณสมบัติความจำเพาะของเอนไซม์ได้เป็นอย่างดี แต่แบบจำลองนี้ไม่สามารถอธิบายความเสถียรของสารในสภาวะทรานซิชัน (transition state) ในขณะที่เอนไซม์เข้าเร่งปฏิกิริยา
แบบจำลองลูกกุญแจและแม่กุญแจได้รับการพิสูจน์แล้วว่าไม่ถูกต้อง ปัจจุบันนิยมใช้แบบจำลองความเหนี่ยวนำให้เหมาะสม (induced fit model) ในการอธิบายลักษณะและการทำงานของเอนไซม์รวมทำโคแฟ็กเตอร์ของเอนไซม์ด้วย
แบบจำลองความเหนี่ยวนำให้เหมาะสม[แก้]

ในปี ค.ศ. 1958 แดเนียล กอซแลนด์ (Daniel Koshland) ได้เสนอแบบจำลองที่ปรับปรุงมาจากแบบจำลองลูกกุญและแม่กุญแจ เนื่องมาจากในเวลาต่อมาพบว่าเอนไซม์มีโครงสร้างที่ยืดหยุ่น และบริเวณเร่ง (active site) สามารถเปลี่ยนรูปร่างได้ระหว่างการทำปฏิกิริยา ขึ้นอยู่กับสารตั้งต้นที่เข้าทำปฏิกิริยากับเอนไซม์[28] As a result, the substrate does not simply bind to a rigid active site; the amino acid side chains which make up the active site are moulded into the precise positions that enable the enzyme to perform its catalytic function. In some cases, such as glycosidases, the substrate molecule also changes shape slightly as it enters the active site.[29] The active site continues to change until the substrate is completely bound, at which point the final shape and charge is determined.[30]
Mechanisms[แก้]
Enzymes can act in several ways, all of which lower ΔG‡:[31]
- Lowering the activation energy by creating an environment in which the transition state is stabilized (e.g. straining the shape of a substrate—by binding the transition-state conformation of the substrate/product molecules, the enzyme distorts the bound substrate(s) into their transition state form, thereby reducing the amount of energy required to complete the transition).
- Lowering the energy of the transition state, but without distorting the substrate, by creating an environment with the opposite charge distribution to that of the transition state.
- Providing an alternative pathway. For example, temporarily reacting with the substrate to form an intermediate ES complex, which would be impossible in the absence of the enzyme.
- Reducing the reaction entropy change by bringing substrates together in the correct orientation to react. Considering ΔH‡ alone overlooks this effect.
Interestingly, this entropic effect involves destabilization of the ground state,[32] and its contribution to catalysis is relatively small.[33]
Transition State Stabilization[แก้]
The understanding of the origin of the reduction of ΔG‡ requires one to find out how the enzymes can stabilize its transition state more than the transition state of the uncatalyzed reaction. Apparently, the most effective way for reaching large stabilization is the use of electrostatic effects, in particular, by having a relatively fixed polar environment that is oriented toward the charge distribution of the transition state.[34] Such an environment does not exist in the uncatalyzed reaction in water.
Dynamics and function[แก้]
The internal dynamics of enzymes is connected to their mechanism of catalysis.[35][36][37] Internal dynamics are the movement of parts of the enzyme's structure, such as individual amino acid residues, a group of amino acids, or even an entire protein domain. These movements occur at various time-scales ranging from femtoseconds to seconds. Networks of protein residues throughout an enzyme's structure can contribute to catalysis through dynamic motions.[38][39][40][41] Protein motions are vital to many enzymes, but whether small and fast vibrations, or larger and slower conformational movements are more important depends on the type of reaction involved. However, although these movements are important in binding and releasing substrates and products, it is not clear if protein movements help to accelerate the chemical steps in enzymatic reactions.[42] These new insights also have implications in understanding allosteric effects and developing new drugs.
Allosteric modulation[แก้]
Allosteric enzymes change their structure in response to binding of effectors. Modulation can be direct, where the effector binds directly to binding sites in the enzyme, or indirect, where the effector binds to other proteins or protein subunits that interact with the allosteric enzyme and thus influence catalytic activity.
Cofactors and coenzymes[แก้]
Cofactors[แก้]
Some enzymes do not need any additional components to show full activity. However, others require non-protein molecules called cofactors to be bound for activity.[43] Cofactors can be either inorganic (e.g., metal ions and iron-sulfur clusters) or organic compounds (e.g., flavin and heme). Organic cofactors can be either prosthetic groups, which are tightly bound to an enzyme, or coenzymes, which are released from the enzyme's active site during the reaction. Coenzymes include NADH, NADPH and adenosine triphosphate. These molecules act to transfer chemical groups between enzymes.[44]
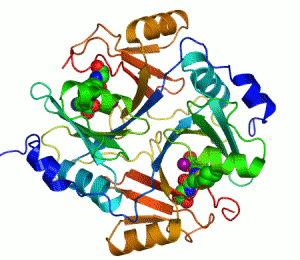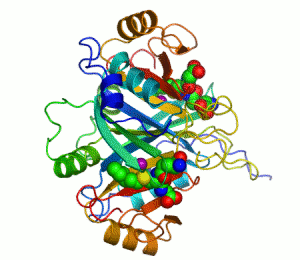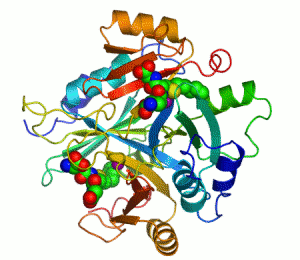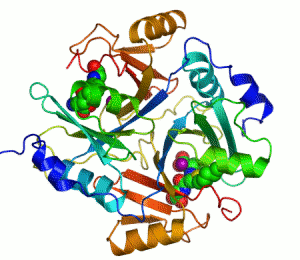
An example of an enzyme that contains a cofactor is carbonic anhydrase, and is shown in the ribbon diagram above with a zinc cofactor bound as part of its active site.[45] These tightly bound molecules are usually found in the active site and are involved in catalysis. For example, flavin and heme cofactors are often involved in redox reactions.
Enzymes that require a cofactor but do not have one bound are called apoenzymes or apoproteins. An apoenzyme together with its cofactor(s) is called a holoenzyme (this is the active form). Most cofactors are not covalently attached to an enzyme, but are very tightly bound. However, organic prosthetic groups can be covalently bound (e.g., thiamine pyrophosphate in the enzyme pyruvate dehydrogenase). The term "holoenzyme" can also be applied to enzymes that contain multiple protein subunits, such as the DNA polymerases, here the holoenzyme is the complete complex containing all the subunits needed for activity.
Coenzymes[แก้]
Coenzymes are small organic molecules that transport chemical groups from one enzyme to another.[46] Some of these chemicals such as riboflavin, thiamine and folic acid are vitamins (compounds which cannot be synthesized by the body and must be acquired from the diet). The chemical groups carried include the hydride ion (H-) carried by NAD or NADP+, the acetyl group carried by coenzyme A, formyl, methenyl or methyl groups carried by folic acid and the methyl group carried by S-adenosylmethionine.
Since coenzymes are chemically changed as a consequence of enzyme action, it is useful to consider coenzymes to be a special class of substrates, or second substrates, which are common to many different enzymes. For example, about 700 enzymes are known to use the coenzyme NADH.[47]
Coenzymes are usually regenerated and their concentrations maintained at a steady level inside the cell: for example, NADPH is regenerated through the pentose phosphate pathway and S-adenosylmethionine by methionine adenosyltransferase.
Thermodynamics[แก้]

As all catalysts, enzymes do not alter the position of the chemical equilibrium of the reaction. Usually, in the presence of an enzyme, the reaction runs in the same direction as it would without the enzyme, just more quickly. However, in the absence of the enzyme, other possible uncatalyzed, "spontaneous" reactions might lead to different products, because in those conditions this different product is formed faster.
Furthermore, enzymes can couple two or more reactions, so that a thermodynamically favorable reaction can be used to "drive" a thermodynamically unfavorable one. For example, the hydrolysis of ATP is often used to drive other chemical reactions.
Enzymes catalyze the forward and backward reactions equally. They do not alter the equilibrium itself, but only the speed at which it is reached. For example, carbonic anhydrase catalyzes its reaction in either direction depending on the concentration of its reactants.
Nevertheless, if the equilibrium is greatly displaced in one direction, that is, in a very exergonic reaction, the reaction is effectively irreversible. Under these conditions the enzyme will, in fact, only catalyze the reaction in the thermodynamically allowed direction.
Kinetics[แก้]

Enzyme kinetics is the investigation of how enzymes bind substrates and turn them into products. The rate data used in kinetic analyses are obtained from enzyme assays.
In 1902 Victor Henri[48] proposed a quantitative theory of enzyme kinetics, but his experimental data were not useful because the significance of the hydrogen ion concentration was not yet appreciated. After Peter Lauritz Sørensen had defined the logarithmic pH-scale and introduced the concept of buffering in 1909[49] the German chemist Leonor Michaelis and his Canadian postdoc Maud Leonora Menten repeated Henri's experiments and confirmed his equation which is referred to as Henri-Michaelis-Menten kinetics (sometimes also Michaelis-Menten kinetics).[50] Their work was further developed by G. E. Briggs and J. B. S. Haldane, who derived kinetic equations that are still widely used today.[51]
The major contribution of Henri was to think of enzyme reactions in two stages. In the first, the substrate binds reversibly to the enzyme, forming the enzyme-substrate complex. This is sometimes called the Michaelis complex. The enzyme then catalyzes the chemical step in the reaction and releases the product.
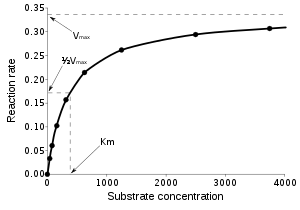
Enzymes can catalyze up to several million reactions per second. For example, the reaction catalyzed by orotidine 5'-phosphate decarboxylase will consume half of its substrate in 78 million years if no enzyme is present. However, when the decarboxylase is added, the same process takes just 25 milliseconds.[52] Enzyme rates depend on solution conditions and substrate concentration. Conditions that denature the protein abolish enzyme activity, such as high temperatures, extremes of pH or high salt concentrations, while raising substrate concentration tends to increase activity. To find the maximum speed of an enzymatic reaction, the substrate concentration is increased until a constant rate of product formation is seen. This is shown in the saturation curve on the right. Saturation happens because, as substrate concentration increases, more and more of the free enzyme is converted into the substrate-bound ES form. At the maximum velocity (Vmax) of the enzyme, all the enzyme active sites are bound to substrate, and the amount of ES complex is the same as the total amount of enzyme. However, Vmax is only one kinetic constant of enzymes. The amount of substrate needed to achieve a given rate of reaction is also important. This is given by the Michaelis-Menten constant (Km), which is the substrate concentration required for an enzyme to reach one-half its maximum velocity. Each enzyme has a characteristic Km for a given substrate, and this can show how tight the binding of the substrate is to the enzyme. Another useful constant is kcat, which is the number of substrate molecules handled by one active site per second.
The efficiency of an enzyme can be expressed in terms of kcat/Km. This is also called the specificity constant and incorporates the rate constants for all steps in the reaction. Because the specificity constant reflects both affinity and catalytic ability, it is useful for comparing different enzymes against each other, or the same enzyme with different substrates. The theoretical maximum for the specificity constant is called the diffusion limit and is about 108 to 109 (M-1 s-1). At this point every collision of the enzyme with its substrate will result in catalysis, and the rate of product formation is not limited by the reaction rate but by the diffusion rate. Enzymes with this property are called catalytically perfect or kinetically perfect. Example of such enzymes are triose-phosphate isomerase, carbonic anhydrase, acetylcholinesterase, catalase, fumarase, β-lactamase, and superoxide dismutase.
Michaelis-Menten kinetics relies on the law of mass action, which is derived from the assumptions of free diffusion and thermodynamically driven random collision. However, many biochemical or cellular processes deviate significantly from these conditions, because of macromolecular crowding, phase-separation of the enzyme/substrate/product, or one or two-dimensional molecular movement.[53] In these situations, a fractal Michaelis-Menten kinetics may be applied.[54][55][56][57]
Some enzymes operate with kinetics which are faster than diffusion rates, which would seem to be impossible. Several mechanisms have been invoked to explain this phenomenon. Some proteins are believed to accelerate catalysis by drawing their substrate in and pre-orienting them by using dipolar electric fields. Other models invoke a quantum-mechanical tunneling explanation, whereby a proton or an electron can tunnel through activation barriers, although for proton tunneling this model remains somewhat controversial.[58][59] Quantum tunneling for protons has been observed in tryptamine.[60] This suggests that enzyme catalysis may be more accurately characterized as "through the barrier" rather than the traditional model, which requires substrates to go "over" a lowered energy barrier.
Inhibition[แก้]
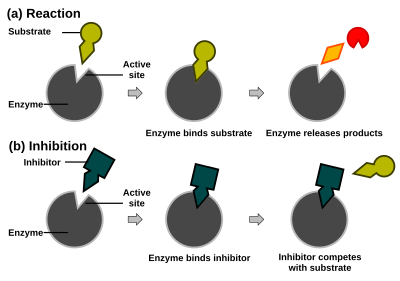

Enzyme reaction rates can be decreased by various types of enzyme inhibitors.
- Competitive inhibition
In competitive inhibition, the inhibitor and substrate compete for the enzyme (i.e., they can not bind at the same time). Often competitive inhibitors strongly resemble the real substrate of the enzyme. For example, methotrexate is a competitive inhibitor of the enzyme dihydrofolate reductase, which catalyzes the reduction of dihydrofolate to tetrahydrofolate. The similarity between the structures of folic acid and this drug are shown in the figure to the right bottom. Note that binding of the inhibitor need not be to the substrate binding site (as frequently stated), if binding of the inhibitor changes the conformation of the enzyme to prevent substrate binding and vice versa. In competitive inhibition the maximal velocity of the reaction is not changed, but higher substrate concentrations are required to reach a given velocity, increasing the apparent Km.
- Uncompetitive inhibition
In uncompetitive inhibition the inhibitor can not bind to the free enzyme, but only to the ES-complex. The EIS-complex thus formed is enzymatically inactive. This type of inhibition is rare, but may occur in multimeric enzymes.
- Non-competitive inhibition
Non-competitive inhibitors can bind to the enzyme at the same time as the substrate, i.e. they never bind to the active site. Both the EI and EIS complexes are enzymatically inactive. Because the inhibitor can not be driven from the enzyme by higher substrate concentration (in contrast to competitive inhibition), the apparent Vmax changes. But because the substrate can still bind to the enzyme, the Km stays the same.
- Mixed inhibition
This type of inhibition resembles the non-competitive, except that the EIS-complex has residual enzymatic activity.
In many organisms inhibitors may act as part of a feedback mechanism. If an enzyme produces too much of one substance in the organism, that substance may act as an inhibitor for the enzyme at the beginning of the pathway that produces it, causing production of the substance to slow down or stop when there is sufficient amount. This is a form of negative feedback. Enzymes which are subject to this form of regulation are often multimeric and have allosteric binding sites for regulatory substances. Their substrate/velocity plots are not hyperbolar, but sigmoidal (S-shaped).
Irreversible inhibitors react with the enzyme and form a covalent adduct with the protein. The inactivation is irreversible. These compounds include eflornithine a drug used to treat the parasitic disease sleeping sickness.[62] Penicillin and Aspirin also act in this manner. With these drugs, the compound is bound in the active site and the enzyme then converts the inhibitor into an activated form that reacts irreversibly with one or more amino acid residues.
- Uses of inhibitors
Since inhibitors modulate the function of enzymes they are often used as drugs. An common example of an inhibitor that is used as a drug is aspirin, which inhibits the COX-1 and COX-2 enzymes that produce the inflammation messenger prostaglandin, thus suppressing pain and inflammation. However, other enzyme inhibitors are poisons. For example, the poison cyanide is an irreversible enzyme inhibitor that combines with the copper and iron in the active site of the enzyme cytochrome c oxidase and blocks cellular respiration.[63]
Biological function[แก้]
Enzymes serve a wide variety of functions inside living organisms. They are indispensable for signal transduction and cell regulation, often via kinases and phosphatases.[64] They also generate movement, with myosin hydrolysing ATP to generate muscle contraction and also moving cargo around the cell as part of the cytoskeleton.[65] Other ATPases in the cell membrane are ion pumps involved in active transport. Enzymes are also involved in more exotic functions, such as luciferase generating light in fireflies.[66] Viruses can also contain enzymes for infecting cells, such as the HIV integrase and reverse transcriptase, or for viral release from cells, like the influenza virus neuraminidase.
An important function of enzymes is in the digestive systems of animals. Enzymes such as amylases and proteases break down large molecules (starch or proteins, respectively) into smaller ones, so they can be absorbed by the intestines. Starch molecules, for example, are too large to be absorbed from the intestine, but enzymes hydrolyse the starch chains into smaller molecules such as maltose and eventually glucose, which can then be absorbed. Different enzymes digest different food substances. In ruminants which have herbivorous diets, microorganisms in the gut produce another enzyme, cellulase to break down the cellulose cell walls of plant fiber.[67]
Several enzymes can work together in a specific order, creating metabolic pathways. In a metabolic pathway, one enzyme takes the product of another enzyme as a substrate. After the catalytic reaction, the product is then passed on to another enzyme. Sometimes more than one enzyme can catalyze the same reaction in parallel, this can allow more complex regulation: with for example a low constant activity being provided by one enzyme but an inducible high activity from a second enzyme.
Enzymes determine what steps occur in these pathways. Without enzymes, metabolism would neither progress through the same steps, nor be fast enough to serve the needs of the cell. Indeed, a metabolic pathway such as glycolysis could not exist independently of enzymes. Glucose, for example, can react directly with ATP to become phosphorylated at one or more of its carbons. In the absence of enzymes, this occurs so slowly as to be insignificant. However, if hexokinase is added, these slow reactions continue to take place except that phosphorylation at carbon 6 occurs so rapidly that if the mixture is tested a short time later, glucose-6-phosphate is found to be the only significant product. Consequently, the network of metabolic pathways within each cell depends on the set of functional enzymes that are present.
Control of activity[แก้]
There are five main ways that enzyme activity is controlled in the cell.
- Enzyme production (transcription and translation of enzyme genes) can be enhanced or diminished by a cell in response to changes in the cell's environment. This form of gene regulation is called enzyme induction and inhibition (see enzyme induction). For example, bacteria may become resistant to antibiotics such as penicillin because enzymes called beta-lactamases are induced that hydrolyse the crucial beta-lactam ring within the penicillin molecule. Another example are enzymes in the liver called cytochrome P450 oxidases, which are important in drug metabolism. Induction or inhibition of these enzymes can cause drug interactions.
- Enzymes can be compartmentalized, with different metabolic pathways occurring in different cellular compartments. For example, fatty acids are synthesized by one set of enzymes in the cytosol, endoplasmic reticulum and the Golgi apparatus and used by a different set of enzymes as a source of energy in the mitochondrion, through β-oxidation.[68]
- Enzymes can be regulated by inhibitors and activators. For example, the end product(s) of a metabolic pathway are often inhibitors for one of the first enzymes of the pathway (usually the first irreversible step, called committed step), thus regulating the amount of end product made by the pathways. Such a regulatory mechanism is called a negative feedback mechanism, because the amount of the end product produced is regulated by its own concentration. Negative feedback mechanism can effectively adjust the rate of synthesis of intermediate metabolites according to the demands of the cells. This helps allocate materials and energy economically, and prevents the manufacture of excess end products. The control of enzymatic action helps to maintain a stable internal environment in living organisms.
- Enzymes can be regulated through post-translational modification. This can include phosphorylation, myristoylation and glycosylation. For example, in the response to insulin, the phosphorylation of multiple enzymes, including glycogen synthase, helps control the synthesis or degradation of glycogen and allows the cell to respond to changes in blood sugar.[69] Another example of post-translational modification is the cleavage of the polypeptide chain. Chymotrypsin, a digestive protease, is produced in inactive form as chymotrypsinogen in the pancreas and transported in this form to the stomach where it is activated. This stops the enzyme from digesting the pancreas or other tissues before it enters the gut. This type of inactive precursor to an enzyme is known as a zymogen.
- Some enzymes may become activated when localized to a different environment (eg. from a reducing (cytoplasm) to an oxidising (periplasm) environment, high pH to low pH etc). For example, hemagglutinin in the influenza virus is activated by a conformational change caused by the acidic conditions, these occur when it is taken up inside its host cell and enters the lysosome.[70]
Involvement in disease[แก้]
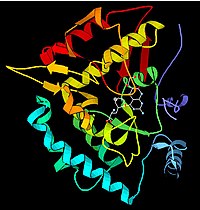
Since the tight control of enzyme activity is essential for homeostasis, any malfunction (mutation, overproduction, underproduction or deletion) of a single critical enzyme can lead to a genetic disease. The importance of enzymes is shown by the fact that a lethal illness can be caused by the malfunction of just one type of enzyme out of the thousands of types present in our bodies.
One example is the most common type of phenylketonuria. A mutation of a single amino acid in the enzyme phenylalanine hydroxylase, which catalyzes the first step in the degradation of phenylalanine, results in build-up of phenylalanine and related products. This can lead to mental retardation if the disease is untreated.[71]
Another example is when germline mutations in genes coding for DNA repair enzymes cause hereditary cancer syndromes such as xeroderma pigmentosum. Defects in these enzymes cause cancer since the body is less able to repair mutations in the genome. This causes a slow accumulation of mutations and results in the development of many types of cancer in the sufferer.
Naming conventions[แก้]
An enzyme's name is often derived from its substrate or the chemical reaction it catalyzes, with the word ending in -ase. Examples are lactase, alcohol dehydrogenase and DNA polymerase. This may result in different enzymes, called isozymes, with the same function having the same basic name. Isoenzymes have a different amino acid sequence and might be distinguished by their optimal pH, kinetic properties or immunologically. Furthermore, the normal physiological reaction an enzyme catalyzes may not be the same as under artificial conditions. This can result in the same enzyme being identified with two different names. E.g. Glucose isomerase, used industrially to convert glucose into the sweetener fructose, is a xylose isomerase in vivo.
The International Union of Biochemistry and Molecular Biology have developed a nomenclature for enzymes, the EC numbers; each enzyme is described by a sequence of four numbers preceded by "EC". The first number broadly classifies the enzyme based on its mechanism:
The top-level classification is
- EC 1 Oxidoreductases: catalyze oxidation/reduction reactions
- EC 2 Transferases: transfer a functional group (e.g. a methyl or phosphate group)
- EC 3 Hydrolases: catalyze the hydrolysis of various bonds
- EC 4 Lyases: cleave various bonds by means other than hydrolysis and oxidation
- EC 5 Isomerases: catalyze isomerization changes within a single molecule
- EC 6 Ligases: join two molecules with covalent bonds
The complete nomenclature can be browsed at http://www.chem.qmul.ac.uk/iubmb/enzyme/.
Industrial applications[แก้]
Enzymes are used in the chemical industry and other industrial applications when extremely specific catalysts are required. However, enzymes in general are limited in the number of reactions they have evolved to catalyze and also by their lack of stability in organic solvents and at high temperatures. Consequently, protein engineering is an active area of research and involves attempts to create new enzymes with novel properties, either through rational design or in vitro evolution.[72][73] These efforts have begun to be successful, and a few enzymes have now been desiged "from scratch" to catalyse reactions that do not occur in nature.[74]
| Application | Enzymes used | Uses |
Food processing  |
Amylases from fungi and plants. | Production of sugars from starch, such as in making high-fructose corn syrup.[75] In baking, catalyze breakdown of starch in the flour to sugar. Yeast fermentation of sugar produces the carbon dioxide that raises the dough. |
| Proteases | Biscuit manufacturers use them to lower the protein level of flour. | |
| Baby foods | Trypsin | To predigest baby foods. |
Brewing industry  |
Enzymes from barley are released during the mashing stage of beer production. | They degrade starch and proteins to produce simple sugar, amino acids and peptides that are used by yeast for fermentation. |
| Industrially produced barley enzymes | Widely used in the brewing process to substitute for the natural enzymes found in barley. | |
| Amylase, glucanases, proteases | Split polysaccharides and proteins in the malt. | |
| Betaglucanases and arabinoxylanases | Improve the wort and beer filtration characteristics. | |
| Amyloglucosidase and pullulanases | Low-calorie beer and adjustment of fermentability. | |
| Proteases | Remove cloudiness produced during storage of beers. | |
| Acetolactatedecarboxylase (ALDC) | Increases fermentation efficiency by reducing diacetyl formation.[76] | |
| Fruit juices | Cellulases, pectinases | Clarify fruit juices |
Dairy industry  |
Rennin, derived from the stomachs of young ruminant animals (like calves and lambs). | Manufacture of cheese, used to hydrolyze protein. |
| Microbially produced enzyme | Now finding increasing use in the dairy industry. | |
| Lipases | Is implemented during the production of Roquefort cheese to enhance the ripening of the blue-mould cheese. | |
| Lactases | Break down lactose to glucose and galactose. | |
| Meat tenderizers | Papain | To soften meat for cooking. |
| Starch industry | Amylases, amyloglucosideases and glucoamylases | Converts starch into glucose and various syrups. |
| Glucose isomerase | Converts glucose into fructose in production of high fructose syrups from starchy materials. These syrups have enhanced sweetening properties and lower calorific values than sucrose for the same level of sweetness. | |
Paper industry |
Amylases, Xylanases, Cellulases and ligninases | Degrade starch to lower viscosity, aiding sizing and coating paper. Xylanases reduce bleach required for decolorising; cellulases smooth fibers, enhance water drainage, and promote ink removal; lipases reduce pitch and lignin-degrading enzymes remove lignin to soften paper. |
Biofuel industry |
Cellulases | Used to break down cellulose into sugars that can be fermented (see cellulosic ethanol). |
| Ligninases | Use of lignin waste | |
| Biological detergent | Primarily proteases, produced in an extracellular form from bacteria | Used for presoak conditions and direct liquid applications helping with removal of protein stains from clothes. |
| Amylases | Detergents for machine dish washing to remove resistant starch residues. | |
| Lipases | Used to assist in the removal of fatty and oily stains. | |
| Cellulases | Used in biological fabric conditioners. | |
| Contact lens cleaners | Proteases | To remove proteins on contact lens to prevent infections. |
| Rubber industry | Catalase | To generate oxygen from peroxide to convert latex into foam rubber. |
| Photographic industry | Protease (ficin) | Dissolve gelatin off scrap film, allowing recovery of its silver content. |
Molecular biology  |
Restriction enzymes, DNA ligase and polymerases | Used to manipulate DNA in genetic engineering, important in pharmacology, agriculture and medicine. Essential for restriction digestion and the polymerase chain reaction. Molecular biology is also important in forensic science. |
See also[แก้]
- List of enzymes
- Enzyme assay
- Enzyme catalysis
- The Proteolysis Map
- RNA Biocatalysis
- SUMO enzymes
- Ki Database
- Proteonomics and protein engineering
- Immobilized enzyme
- Kinetic Perfection
References[แก้]
- ↑ Smith AL (Ed); และคณะ (1997). Oxford dictionary of biochemistry and molecular biology. Oxford [Oxfordshire]: Oxford University Press. ISBN 0-19-854768-4.
{{cite book}}: ใช้ et al. อย่างชัดเจน ใน|author=(help) - ↑ Grisham, Charles M.; Reginald H. Garrett (1999). Biochemistry. Philadelphia: Saunders College Pub. pp. 426–7. ISBN 0-03-022318-0.
{{cite book}}: CS1 maint: multiple names: authors list (ลิงก์) - ↑ Tymoczko, John L.; Stryer Berg Tymoczko; Stryer, Lubert; Berg, Jeremy Mark (2002). Biochemistry. San Francisco: W.H. Freeman. ISBN 0-7167-4955-6.
Until the 1980s, all biological catalysts, termed enzymes, were believed to be proteins. Then, Tom Cech and Sidney Altman independently discovered that certain RNA molecules can be effective catalysts. These RNA catalysts have come to be known as ribozymes.
{{cite book}}: CS1 maint: multiple names: authors list (ลิงก์) - ↑ Bairoch A. (2000). "The ENZYME database in 2000" (PDF). Nucleic Acids Res. 28: 304–5. doi:10.1093/nar/28.1.304. PMID 10592255.
- ↑ Lilley D (2005). "Structure, folding and mechanisms of ribozymes". Curr Opin Struct Biol. 15 (3): 313–23. doi:10.1016/j.sbi.2005.05.002. PMID 15919196.
- ↑ Cech T (2000). "Structural biology. The ribosome is a ribozyme". Science. 289 (5481): 878–9. doi:10.1126/science.289.5481.878. PMID 10960319.
- ↑ Groves JT (1997). "Artificial enzymes. The importance of being selective". Nature. 389 (6649): 329–30. doi:10.1038/38602. PMID 9311771.
- ↑ de Réaumur, RAF (1752). "Observations sur la digestion des oiseaux". Histoire de l'academie royale des sciences. 1752: 266, 461.
- ↑ Williams, H. S. (1904) A History of Science: in Five Volumes. Volume IV: Modern Development of the Chemical and Biological Sciences Harper and Brothers (New York) Accessed 4 April 2007
- ↑ Dubos J. (1951). "Louis Pasteur: Free Lance of Science, Gollancz. Quoted in Manchester K. L. (1995) Louis Pasteur (1822–1895)—chance and the prepared mind". Trends Biotechnol. 13 (12): 511–5. doi:10.1016/S0167-7799(00)89014-9. PMID 8595136.
- ↑ Nobel Laureate Biography of Eduard Buchner at http://nobelprize.org Accessed 4 April 2007
- ↑ Text of Eduard Buchner's 1907 Nobel lecture at http://nobelprize.org Accessed 4 April 2007
- ↑ 1946 Nobel prize for Chemistry laureates at http://nobelprize.org Accessed 4 April 2007
- ↑ Blake CC, Koenig DF, Mair GA, North AC, Phillips DC, Sarma VR. (1965). "Structure of hen egg-white lysozyme. A three-dimensional Fourier synthesis at 2 Angstrom resolution". Nature. 22 (206): 757–61. doi:10.1038/206757a0. PMID 5891407.
{{cite journal}}: CS1 maint: multiple names: authors list (ลิงก์) - ↑ Chen LH, Kenyon GL, Curtin F, Harayama S, Bembenek ME, Hajipour G, Whitman CP (1992). "4-Oxalocrotonate tautomerase, an enzyme composed of 62 amino acid residues per monomer". J. Biol. Chem. 267 (25): 17716–21. PMID 1339435.
{{cite journal}}: CS1 maint: multiple names: authors list (ลิงก์) - ↑ Smith S (1994). "The animal fatty acid synthase: one gene, one polypeptide, seven enzymes". Faseb J. 8 (15): 1248–59. PMID 8001737.
{{cite journal}}: ไม่รู้จักพารามิเตอร์|day=ถูกละเว้น (help); ไม่รู้จักพารามิเตอร์|month=ถูกละเว้น (help) - ↑ Anfinsen C.B. (1973). "Principles that Govern the Folding of Protein Chains". Science. 181: 223–30. doi:10.1126/science.181.4096.223. PMID 4124164.
- ↑ Dunaway-Mariano D (November 2008). "Enzyme function discovery". Structure. 16 (11): 1599–600. doi:10.1016/j.str.2008.10.001. PMID 19000810.
- ↑ The Catalytic Site Atlas at The European Bioinformatics Institute Accessed 4 April 2007
- ↑ Jaeger KE, Eggert T. (2004). "Enantioselective biocatalysis optimized by directed evolution". Curr Opin Biotechnol. 15 (4): 305–13. doi:10.1016/j.copbio.2004.06.007. PMID 15358000.
- ↑ Shevelev IV, Hubscher U. (2002). "The 3' 5' exonucleases". Nat Rev Mol Cell Biol. 3 (5): 364–76. doi:10.1038/nrm804. PMID 11988770.
- ↑ Tymoczko, John L.; Stryer Berg Tymoczko; Stryer, Lubert; Berg, Jeremy Mark (2002). Biochemistry. San Francisco: W.H. Freeman. ISBN 0-7167-4955-6.
{{cite book}}: CS1 maint: multiple names: authors list (ลิงก์) - ↑ Zenkin N, Yuzenkova Y, Severinov K. (2006). "Transcript-assisted transcriptional proofreading". Science. 313: 518–20. doi:10.1126/science.1127422. PMID 16873663.
{{cite journal}}: CS1 maint: multiple names: authors list (ลิงก์) - ↑ Ibba M, Soll D. (2000). "Aminoacyl-tRNA synthesis". Annu Rev Biochem. 69: 617–50. doi:10.1146/annurev.biochem.69.1.617. PMID 10966471.
- ↑ Rodnina MV, Wintermeyer W. (2001). "Fidelity of aminoacyl-tRNA selection on the ribosome: kinetic and structural mechanisms". Annu Rev Biochem. 70: 415–35. doi:10.1146/annurev.biochem.70.1.415. PMID 11395413.
- ↑ Firn, Richard. "The Screening Hypothesis - a new explanation of secondary product diversity and function". สืบค้นเมื่อ 2006-10-11.
- ↑ Fischer E. (1894). "Einfluss der Configuration auf die Wirkung der Enzyme". Ber. Dt. Chem. Ges. 27: 2985–93. doi:10.1002/cber.18940270364.
{{cite journal}}: line feed character ใน|journal=ที่ตำแหน่ง 9 (help) - ↑ Koshland D. E. (1958). "Application of a Theory of Enzyme Specificity to Protein Synthesis". Proc. Natl. Acad. Sci. 44 (2): 98–104. doi:10.1073/pnas.44.2.98. PMID 16590179.
- ↑ Vasella A, Davies GJ, Bohm M. (2002). "Glycosidase mechanisms". Curr Opin Chem Biol. 6 (5): 619–29. doi:10.1016/S1367-5931(02)00380-0. PMID 12413546.
{{cite journal}}: CS1 maint: multiple names: authors list (ลิงก์) - ↑ Boyer, Rodney (2002) [2002]. "6". Concepts in Biochemistry (2nd ed.). New York, Chichester, Weinheim, Brisbane, Singapore, Toronto.: John Wiley & Sons, Inc. pp. 137–8. ISBN 0-470-00379-0. OCLC 51720783.
{{cite book}}:|access-date=ต้องการ|url=(help) - ↑ Fersht, Alan (1985). Enzyme structure and mechanism. San Francisco: W.H. Freeman. pp. 50–2. ISBN 0-7167-1615-1.
- ↑ Jencks, William P. (1987). Catalysis in chemistry and enzymology. Mineola, N.Y: Dover. ISBN 0-486-65460-5.
- ↑ Villa J, Strajbl M, Glennon TM, Sham YY, Chu ZT, Warshel A (2000). "How important are entropic contributions to enzyme catalysis?". Proc. Natl. Acad. Sci. U.S.A. 97 (22): 11899–904. doi:10.1073/pnas.97.22.11899. PMID 11050223.
{{cite journal}}: CS1 maint: multiple names: authors list (ลิงก์) - ↑ Warshel A, Sharma PK, Kato M, Xiang Y, Liu H, Olsson MH (2006). "Electrostatic basis for enzyme catalysis". Chem. Rev. 106 (8): 3210–35. doi:10.1021/cr0503106. PMID 16895325.
{{cite journal}}: CS1 maint: multiple names: authors list (ลิงก์) - ↑ Eisenmesser EZ, Bosco DA, Akke M, Kern D (February 2002). "Enzyme dynamics during catalysis". Science. 295 (5559): 1520–3. doi:10.1126/science.1066176. PMID 11859194.
{{cite journal}}: CS1 maint: multiple names: authors list (ลิงก์) - ↑ Agarwal PK (November 2005). "Role of protein dynamics in reaction rate enhancement by enzymes". J. Am. Chem. Soc. 127 (43): 15248–56. doi:10.1021/ja055251s. PMID 16248667.
- ↑ Eisenmesser EZ, Millet O, Labeikovsky W; และคณะ (November 2005). "Intrinsic dynamics of an enzyme underlies catalysis". Nature. 438 (7064): 117–21. doi:10.1038/nature04105. PMID 16267559.
{{cite journal}}: ใช้ et al. อย่างชัดเจน ใน|author=(help)CS1 maint: multiple names: authors list (ลิงก์) - ↑ Yang LW, Bahar I (5 June 2005). "Coupling between catalytic site and collective dynamics: A requirement for mechanochemical activity of enzymes". Structure. 13: 893–904. doi:10.1016/j.str.2005.03.015. PMID 15939021.
- ↑ Agarwal PK, Billeter SR, Rajagopalan PT, Benkovic SJ, Hammes-Schiffer S. (5 March 2002). "Network of coupled promoting motions in enzyme catalysis". Proc Natl Acad Sci USA. 99: 2794–9. doi:10.1073/pnas.052005999. PMID 11867722.
{{cite journal}}: CS1 maint: multiple names: authors list (ลิงก์) - ↑ Agarwal PK, Geist A, Gorin A (August 2004). "Protein dynamics and enzymatic catalysis: investigating the peptidyl-prolyl cis-trans isomerization activity of cyclophilin A". Biochemistry. 43 (33): 10605–18. doi:10.1021/bi0495228. PMID 15311922.
{{cite journal}}: CS1 maint: multiple names: authors list (ลิงก์) - ↑ Tousignant A, Pelletier JN. (August 2004). "Protein motions promote catalysis". Chem Biol. 11 (8): 1037–42. doi:10.1016/j.chembiol.2004.06.007. PMID 15324804.
- ↑ Olsson MHM, Parson WW, Warshel A (2006). "Dynamical Contributions to Enzyme Catalysis: Critical Tests of A Popular Hypothesis". Chem. Rev. 106 (5): 1737–56. doi:10.1021/cr040427e.
{{cite journal}}: CS1 maint: multiple names: authors list (ลิงก์) - ↑ de Bolster, M.W.G. (1997). "Glossary of Terms Used in Bioinorganic Chemistry: Cofactor". International Union of Pure and Applied Chemistry. สืบค้นเมื่อ 2007-10-30.
- ↑ de Bolster, M.W.G. (1997). "Glossary of Terms Used in Bioinorganic Chemistry: Coenzyme". International Union of Pure and Applied Chemistry. สืบค้นเมื่อ 2007-10-30.
- ↑ Fisher Z, Hernandez Prada JA, Tu C, Duda D, Yoshioka C, An H, Govindasamy L, Silverman DN and McKenna R. (2005). "Structural and kinetic characterization of active-site histidine as a proton shuttle in catalysis by human carbonic anhydrase II". Biochemistry. 44 (4): 1097–115. doi:10.1021/bi0480279. PMID 15667203.
{{cite journal}}: CS1 maint: multiple names: authors list (ลิงก์) - ↑ Wagner, Arthur L. (1975). Vitamins and Coenzymes. Krieger Pub Co. ISBN 0-88275-258-8.
- ↑ BRENDA The Comprehensive Enzyme Information System Accessed 4 April 2007
- ↑ Henri, V. (1902). "Theorie generale de l'action de quelques diastases". Compt. Rend. Hebd. Acad. Sci. Paris. 135: 916–9.
- ↑ Sørensen,P.L. (1909). "Enzymstudien {II}. Über die Messung und Bedeutung der Wasserstoffionenkonzentration bei enzymatischen Prozessen". Biochem. Z. 21: 131–304.
- ↑ Michaelis L., Menten M. (1913). "Die Kinetik der Invertinwirkung". Biochem. Z. 49: 333–369. English translation Accessed 6 April 2007
- ↑ Briggs G. E., Haldane J. B. S. (1925). "A note on the kinetics of enzyme action". Biochem. J. 19: 339–339. PMID 16743508.
- ↑ Radzicka A, Wolfenden R. (1995). "A proficient enzyme". Science. 6 (267): 90–931. doi:10.1126/science.7809611. PMID 7809611.
- ↑ Ellis RJ (2001). "Macromolecular crowding: obvious but underappreciated". Trends Biochem. Sci. 26 (10): 597–604. doi:10.1016/S0968-0004(01)01938-7. PMID 11590012.
- ↑ Kopelman R (1988). "Fractal Reaction Kinetics". Science. 241 (4873): 1620–26. doi:10.1126/science.241.4873.1620. PMID 17820893.
- ↑ Savageau MA (1995). "Michaelis-Menten mechanism reconsidered: implications of fractal kinetics". J. Theor. Biol. 176 (1): 115–24. doi:10.1006/jtbi.1995.0181. PMID 7475096.
- ↑ Schnell S, Turner TE (2004). "Reaction kinetics in intracellular environments with macromolecular crowding: simulations and rate laws". Prog. Biophys. Mol. Biol. 85 (2–3): 235–60. doi:10.1016/j.pbiomolbio.2004.01.012. PMID 15142746.
- ↑ Xu F, Ding H (2007). "A new kinetic model for heterogeneous (or spatially confined) enzymatic catalysis: Contributions from the fractal and jamming (overcrowding) effects". Appl. Catal. A: Gen. 317 (1): 70–81. doi:10.1016/j.apcata.2006.10.014.
- ↑ Garcia-Viloca M., Gao J., Karplus M., Truhlar D. G. (2004). "How enzymes work: analysis by modern rate theory and computer simulations". Science. 303 (5655): 186–95. doi:10.1126/science.1088172. PMID 14716003.
{{cite journal}}: CS1 maint: multiple names: authors list (ลิงก์) - ↑
Olsson M. H., Siegbahn P. E., Warshel A. (2004). "Simulations of the large kinetic isotope effect and the temperature dependence of the hydrogen atom transfer in lipoxygenase". J. Am. Chem. Soc. 126 (9): 2820–8. doi:10.1021/ja037233l. PMID 14995199.
{{cite journal}}: CS1 maint: multiple names: authors list (ลิงก์) - ↑ Masgrau L., Roujeinikova A., Johannissen L. O., Hothi P., Basran J., Ranaghan K. E., Mulholland A. J., Sutcliffe M. J., Scrutton N. S., Leys D. (2006). "Atomic Description of an Enzyme Reaction Dominated by Proton Tunneling". Science. 312 (5771): 237–41. doi:10.1126/science.1126002. PMID 16614214.
{{cite journal}}: CS1 maint: multiple names: authors list (ลิงก์) - ↑ Cleland, W.W. (1963). "The Kinetics of Enzyme-catalyzed Reactions with two or more Substrates or Products 2. {I}nhibition: Nomenclature and Theory". Biochim. Biophys. Acta. 67: 173–87.
- ↑ Poulin R, Lu L, Ackermann B, Bey P, Pegg AE. Mechanism of the irreversible inactivation of mouse ornithine decarboxylase by alpha-difluoromethylornithine. Characterization of sequences at the inhibitor and coenzyme binding sites. J Biol Chem. 1992 January 5;267(1):150–8. PMID 1730582
- ↑ Yoshikawa S and Caughey WS. (May 1990). "Infrared evidence of cyanide binding to iron and copper sites in bovine heart cytochrome c oxidase. Implications regarding oxygen reduction". J Biol Chem. 265 (14): 7945–58. PMID 2159465.
{{cite journal}}: ไม่รู้จักพารามิเตอร์|day=ถูกละเว้น (help) - ↑ Hunter T. (1995). "Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling". Cell. 80 (2): 225–36. doi:10.1016/0092-8674(95)90405-0. PMID 7834742.
- ↑ Berg JS, Powell BC, Cheney RE (April 2001). "A millennial myosin census". Mol. Biol. Cell. 12 (4): 780–94. PMC 32266. PMID 11294886.
{{cite journal}}: ไม่รู้จักพารามิเตอร์|day=ถูกละเว้น (help)CS1 maint: multiple names: authors list (ลิงก์) - ↑ Meighen EA (March 1991). "Molecular biology of bacterial bioluminescence". Microbiol. Rev. 55 (1): 123–42. PMC 372803. PMID 2030669.
{{cite journal}}: ไม่รู้จักพารามิเตอร์|day=ถูกละเว้น (help) - ↑ Mackie RI, White BA (1990). "Recent advances in rumen microbial ecology and metabolism: potential impact on nutrient output". J. Dairy Sci. 73 (10): 2971–95. PMID 2178174.
{{cite journal}}: ไม่รู้จักพารามิเตอร์|day=ถูกละเว้น (help); ไม่รู้จักพารามิเตอร์|month=ถูกละเว้น (help) - ↑ Faergeman NJ, Knudsen J (April 1997). "Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling". Biochem. J. 323 (Pt 1): 1–12. PMC 1218279. PMID 9173866.
- ↑ Doble B. W., Woodgett J. R. (April 2003). "GSK-3: tricks of the trade for a multi-tasking kinase". J. Cell. Sci. 116: 1175–86. doi:10.1242/jcs.00384. PMID 12615961.
- ↑ Carr C. M., Kim P. S. (April 2003). "A spring-loaded mechanism for the conformational change of influenza hemagglutinin". Cell. 73: 823–32. doi:10.1016/0092-8674(93)90260-W. PMID 8500173.
- ↑ Phenylketonuria: NCBI Genes and Disease Accessed 4 April 2007
- ↑ Renugopalakrishnan V, Garduno-Juarez R, Narasimhan G, Verma CS, Wei X, Li P. (2005). "Rational design of thermally stable proteins: relevance to bionanotechnology". J Nanosci Nanotechnol. 5 (11): 1759–1767. doi:10.1166/jnn.2005.441. PMID 16433409.
{{cite journal}}: CS1 maint: multiple names: authors list (ลิงก์) - ↑ Hult K, Berglund P. (2003). "Engineered enzymes for improved organic synthesis". Curr Opin Biotechnol. 14 (4): 395–400. doi:10.1016/S0958-1669(03)00095-8. PMID 12943848.
- ↑ Jiang L, Althoff EA, Clemente FR; และคณะ (March 2008). "De novo computational design of retro-aldol enzymes". Science (journal). 319 (5868): 1387–91. doi:10.1126/science.1152692. PMID 18323453.
{{cite journal}}: ใช้ et al. อย่างชัดเจน ใน|author=(help)CS1 maint: multiple names: authors list (ลิงก์) - ↑ Guzmán-Maldonado H, Paredes-López O (September 1995). "Amylolytic enzymes and products derived from starch: a review". Critical reviews in food science and nutrition. 35 (5): 373–403. PMID 8573280.
- ↑ Dulieu C, Moll M, Boudrant J, Poncelet D (2000). "Improved performances and control of beer fermentation using encapsulated alpha-acetolactate decarboxylase and modeling". Biotechnology progress. 16 (6): 958–65. doi:10.1021/bp000128k. PMID 11101321.
{{cite journal}}: CS1 maint: multiple names: authors list (ลิงก์)
Further reading[แก้]
|
Etymology and history
Enzyme structure and mechanism
Thermodynamics
|
Kinetics and inhibition
Function and control of enzymes in the cell
Enzyme-naming conventions
Industrial applications
|
External links[แก้]
- Structure/Function of Enzymes, Web tutorial on enzyme structure and function.
- UC Berkeley video lecture on enzyme structure and function
- Enzymes in diagnosis Role of enzymes in diagnosis of diseases.
- Enzyme spotlight Monthly feature at the European Bioinformatics Institute on a selected enzyme.
- AMFEP, Association of Manufacturers and Formulators of Enzyme Products
- BRENDA database, a comprehensive compilation of information and literature references about all known enzymes; requires payment by commercial users.
- Enzyme Structures Database links to the known 3-D structure data of enzymes in the Protein Data Bank.
- ExPASy enzyme database, links to Swiss-Prot sequence data, entries in other databases and to related literature searches.
- KEGG: Kyoto Encyclopedia of Genes and Genomes Graphical and hypertext-based information on biochemical pathways and enzymes.
- MACiE database of enzyme reaction mechanisms.
- MetaCyc database of enzymes and metabolic pathways
- Face-to-Face Interview with Sir John Cornforth who was awarded a Nobel Prize for work on stereochemistry of enzyme-catalyzed reactions Freeview video by the Vega Science Trust
- Sigma Aldrich Enzyme Assays by Enzyme Name – Hundreds of assays sorted by enzyme name.
- Bugg TD (October 2001). "The development of mechanistic enzymology in the 20th century". Nat Prod Rep. 18 (5): 465–93. doi:10.1039/b009205n. PMID 11699881.